
SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold Immunochromatography) for the detection of novel coronavirus (SARS-CoV-2) antigen in oropharyngeal swab and nasopharyngeal swab specimen, using a sandwich method with double antibodies, is one of the main methods for detecting SARS-CoV-2 infections.
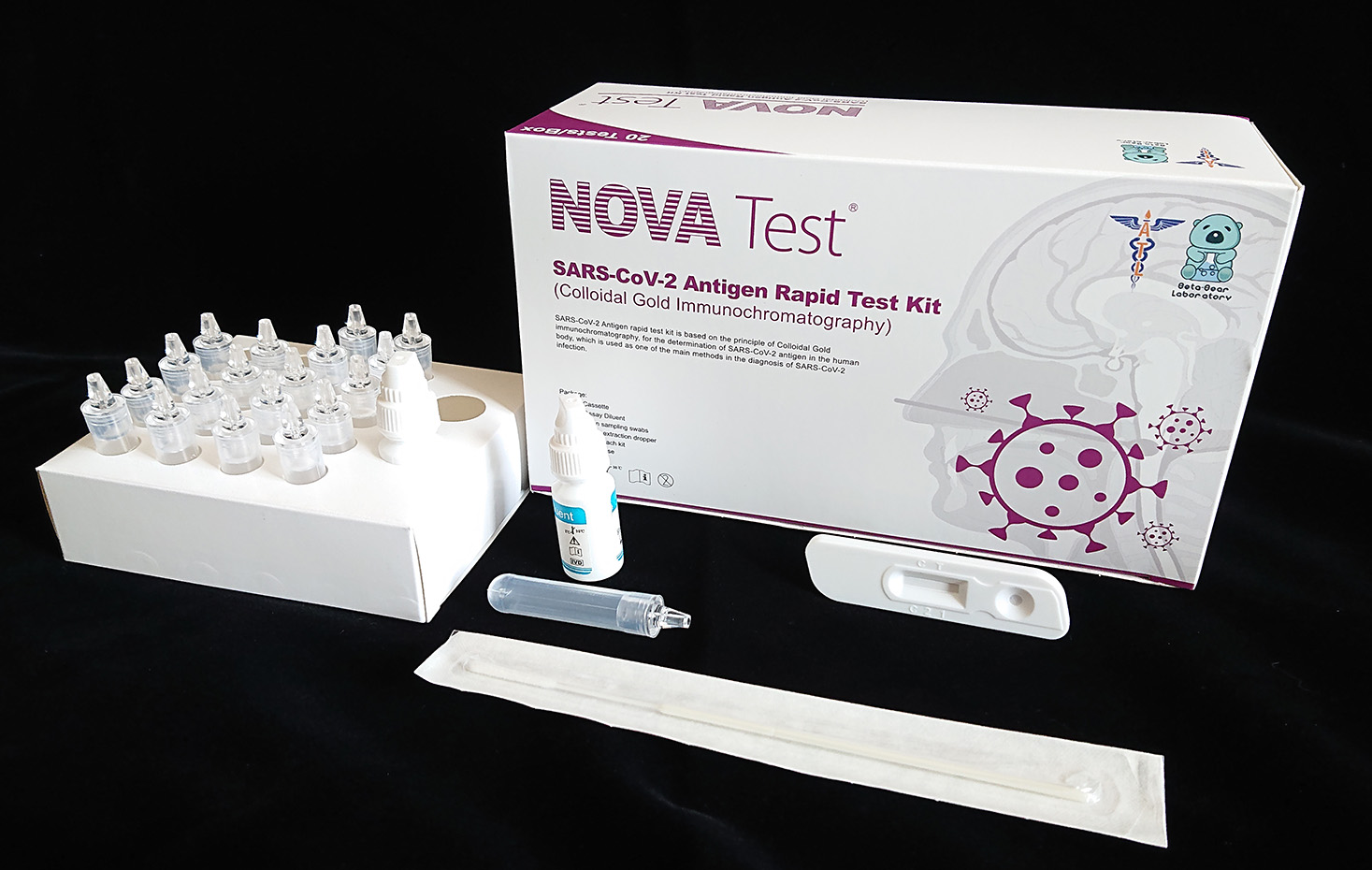
This product is easy to operate, provides rapid detection, and is affordable, making it suitable for the rapid screening of suspected patients and key populations, and can assist in early diagnosis and subsequent treatment. From its inception, Atlas Link Biotech has always maintained a rigorous scientific attitude and professional production processes, and has been committed to the research and production of in vitro diagnostics. We will always rely on technological innovation as the driving force for our business development, and strive to make testing for various diseases simpler, faster, and more affordable. Providing high-quality products at competitive prices to meet customer needs is our consistent commitment.